Recall of Common Anxiety Medication Expanded Over “Life-Threatening” Mistake, FDA Says

Anxiety disorders are challenging diagnoses, causing symptoms like intense fear, discomfort, and panic attacks that can interfere with your daily life. Still, these disorders are also fairly common, with nearly 20 percent of U.S. adults reporting an anxiety disorder, according to the National Institute of Mental Health. Thankfully, there are medications to treat these conditions, including antidepressants, benzodiazepines, and beta blockers. But if you’re prescribed something for anxiety, you’ll want to be aware of a recent notice from the U.S. Food and Drug Administration (FDA) expanding an existing recall.
RELATED: Common Anxiety Medication Recalled Over “Life-Threatening” Mistake, FDA Says.
Over the summer, Pennsylvania-based Endo, Inc. announced a voluntary recall of one lot of Clonazepam Orally Disintegrating Tablets, USP (C-IV) tablets, which are indicated to treat seizures and panic disorder. The meds were pulled “due to mislabeling where an incorrect strength appears on the cartons of some packs,” a July 17 FDA notice explains. While some packages contained 0.25 mg tablets, the cartons said they contained 0.125 mg tablets. The blister packs within the cartons had the accurate strength listed (0.25 mg).
According to the FDA, the error was made by a third-party packager. While the recall was initially limited to a solitary lot, a Nov. 19 announcement confirmed that Endo, USA, Inc. (an Endo subsidiary) expanded it to include 16 lots. This was again due to potential mislabeling, including packages containing 2 mg tablets that showed 1 mg on the carton.
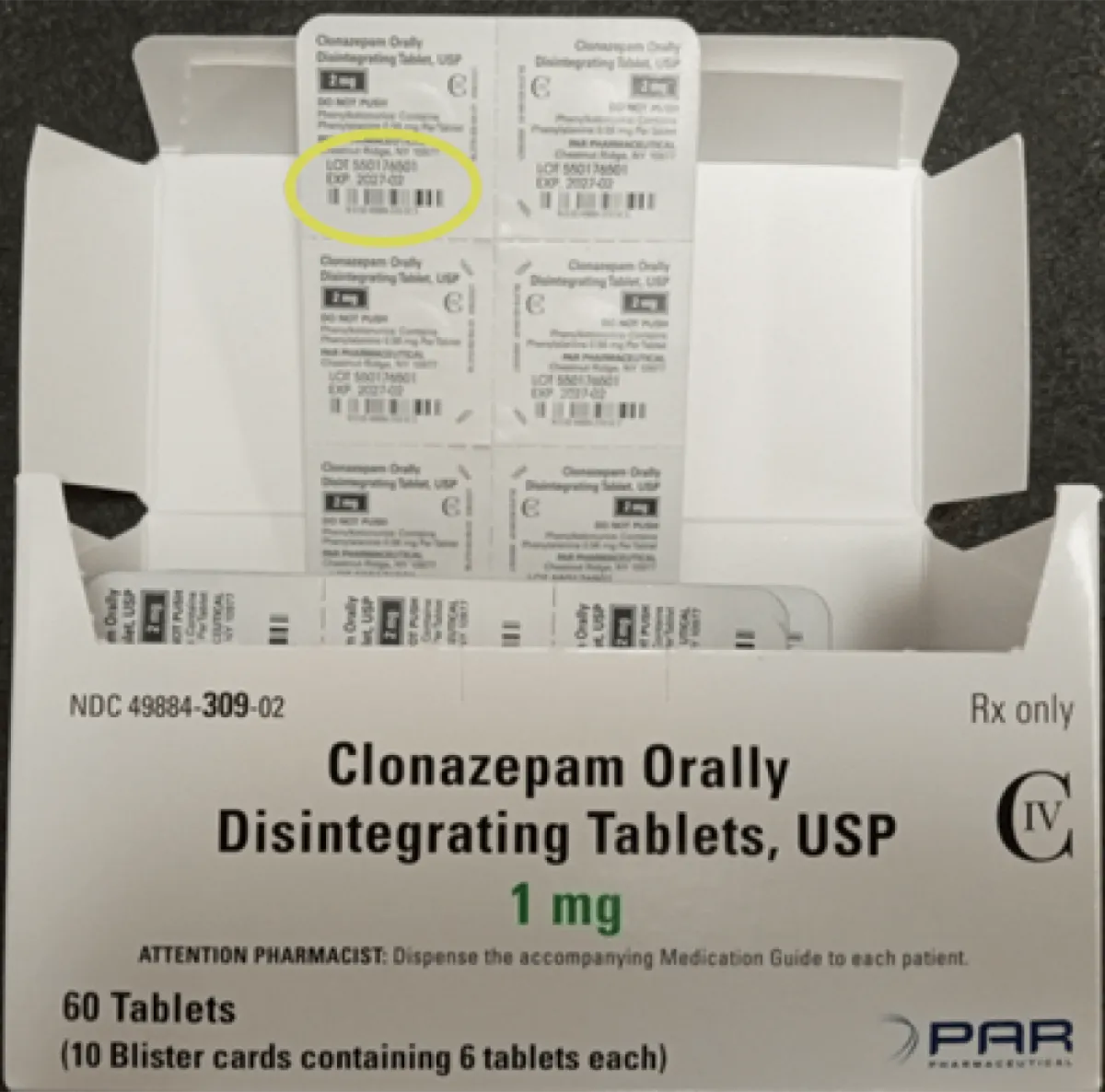
An investigation currently being conducted by Enzo found that more clonazepam product lots could have the incorrect strength on the carton, as well as an incorrect National Drug Code (NDC). The blister packs still reflect the accurate strength.
The latest FDA notice includes a full list of affected lots and NDC numbers, but you can also identify recalled products by their packaging. Each carton contains 60 tablets packed in 10 blister strips (each containing six tablets). The packaging lists Par Parhamceutical, which “previously marketed clonazepam before the product was acquired by Endo,” the notice reads.
RELATED: 44,000 Ovens Are Being Recalled Due to Burn and Fire Hazards.
If you have unused prescribed tablets included in the recall, the FDA advises you to stop using them due to potential health risks.
“Children and adults who inadvertently consume a higher dose of clonazepam could be at increased risk for the adverse events of significant sedation, confusion, dizziness, diminished reflexes, ataxia, and hypotonia,” the FDA notice reads.
The agency continues, “There is reasonable probability for significant, possibly life-threatening, respiratory depression especially for patients with concomitant pulmonary disease, patients who have prescribed dosing near maximal dosing, and patients also taking other medications that could cause additional respiratory depression.”
If you took the wrong dose by accident, the FDA also asks that you talk to your doctor.