Ozempic Competitor Mounjaro Is Becoming Even More Popular—Here's Why
The active ingredient in Mounjaro, tirzepatide, could become a major bestseller, experts say.
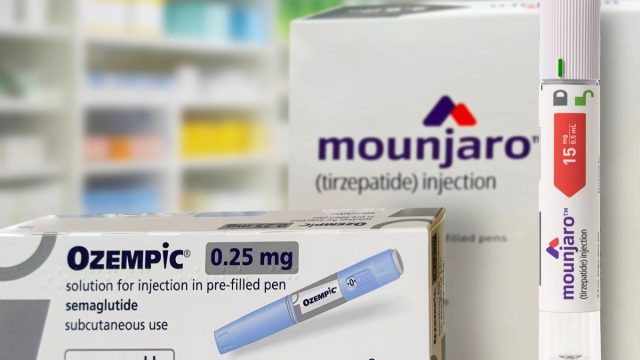
In terms of weight-loss drugs, Novo Nordisk's type 2 diabetes treatment Ozempic—and its sister drug for weight loss, Wegovy—are typically the first that come to mind. But in light of these medications' rise as a popular solution for shedding pounds, competitors want a share of the market. Eli Lilly now has comparable options in the form of Mounjaro (approved for type 2 diabetes) and Zepbound (approved for weight loss), both of which contain the same active ingredient, tirzepatide. And while semaglutide—the active ingredient in both Wegovy and Ozempic—has dominated the market, tirzepatide is becoming even more popular.
RELATED: Ozempic Patients Say It "Stops Working" for Weight Loss—How to Prevent That.
Tirzepatide was approved by the U.S. Food and Drug Administration (FDA) for type 2 diabetes under the trade name Mounjaro in 2022, but the drug is often prescribed off-label for weight loss, just like Ozempic. Last month, tirzepatide was also approved for chronic weight management under the brand name Zepbound.
To assist with weight loss, the drug activates two hormone receptors called glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP) to reduce hunger and food intake, per the FDA. This differs from Ozempic and Wegovy, which only mimic the effects of one hormone, GLP-1.
Tirzepatide has since set itself apart from semaglutide treatments, with new research suggesting it's more effective in a study conducted by Truveta Research. Released on Nov. 22 (and not yet peer-reviewed), the study found that after a year of treatment, patients taking tirzepatide were "significantly more likely" to achieve 5 percent, 10 percent, and 15 percent weight loss when compared with those taking semaglutide. They were also more likely to lose weight at the three-month, six-month, and 12-month marks.
Regarding diabetes, another study funded by Eli Lilly found that tirzepatide led to greater reductions in blood sugar levels in addition to more weight loss when compared with semaglutide, The New York Times reported. (It's worth noting that the study did compare different doses.)
In light of promising results, next year alone, Morgan Stanley predicts that Zepbound will make $2.2 billion in sales, while Bank of America puts that number even higher, at $2.7 billion, CNBC reported. By 2029, Mounjaro is anticipated to have sales totaling $27 billion, accounting for 36 percent growth, per BioPharmaReporter. This dwarfs the projection for Ozempic over the next seven years, as the drug is anticipated to grow by 6.5 percent.
As CNBC reported, Wall Street is excited about Zepbound because it could cause more weight loss than Wegovy, but data comparing the two directly is needed to confirm that. Reuters reported that a head-to-head trial comparing Zepbound and Wegovy in patients with obesity or who are overweight is being conducted by Eli Lilly, and results are expected in 2025.
RELATED: Ozempic Patient Reveals "Repulsive" New Side Effect.
Speaking with BioPharmaReporter Kevin Marcaida, pharma analyst at GlobalData, said that in the short term, we can still expect Ozempic to lead sales, as it was approved five years before Mounjaro. However, by 2027, Mounjaro should move past Ozempic thanks to its demonstrated clinical efficacy. The growth will also be aided by tirzepatide's recent approval for weight loss under the Zepbound brand name, the outlet reported.
Beyond just outpacing Ozempic, Marcaida anticipates that Mounjaro/Zepbound will best other brands as well—leading sales in the diabetes and obesity market by 2029. Seamus Fernandez, a Guggenheim analyst, offered a similar prediction to Marcaida's, telling CNBC that tirzepatide has a "very strong shot of being the best-selling molecule of all time in the pharmaceutical industry."
But that's not to say that other brands won't see growth as well. According to CNBC, Wegovy may be on the way up if it receives approval for expanded use for heart health in the U.S. and Europe, CNBC reported. If awarded, it would be the first GLP-1 drug to receive this approval, Eduardo Grunvald, MD, medical director for UC San Diego's Center for Advanced Weight Management, told the outlet. Beyond that, it could put added pressure on insurance companies to cover these kinds of treatments.
In total, analysts anticipate the overall weight-loss drug market will continue to expand, eventually reaching $100 billion by 2030, CNBC reported. Those from Goldman Sachs also predict that roughly 15 million U.S. adults will be taking these medications within the same timeframe.
RELATED: For more up-to-date information, sign up for our daily newsletter.
Best Life offers the most up-to-date information from top experts, new research, and health agencies, but our content is not meant to be a substitute for professional guidance. When it comes to the medication you're taking or any other health questions you have, always consult your healthcare provider directly.
- Source: FDA Approves New Medication for Chronic Weight Management
- Source: Comparative Effectiveness of Semaglutide and Tirzepatide for Weight Loss in Adults with Overweight and Obesity in the US: A Real-World Evidence Study
- Source: Tirzepatide versus Semaglutide Once Weekly in Patients with Type 2 Diabetes