FDA Approves New Weight Loss Drug That Slashes Patients' Weight by 18%
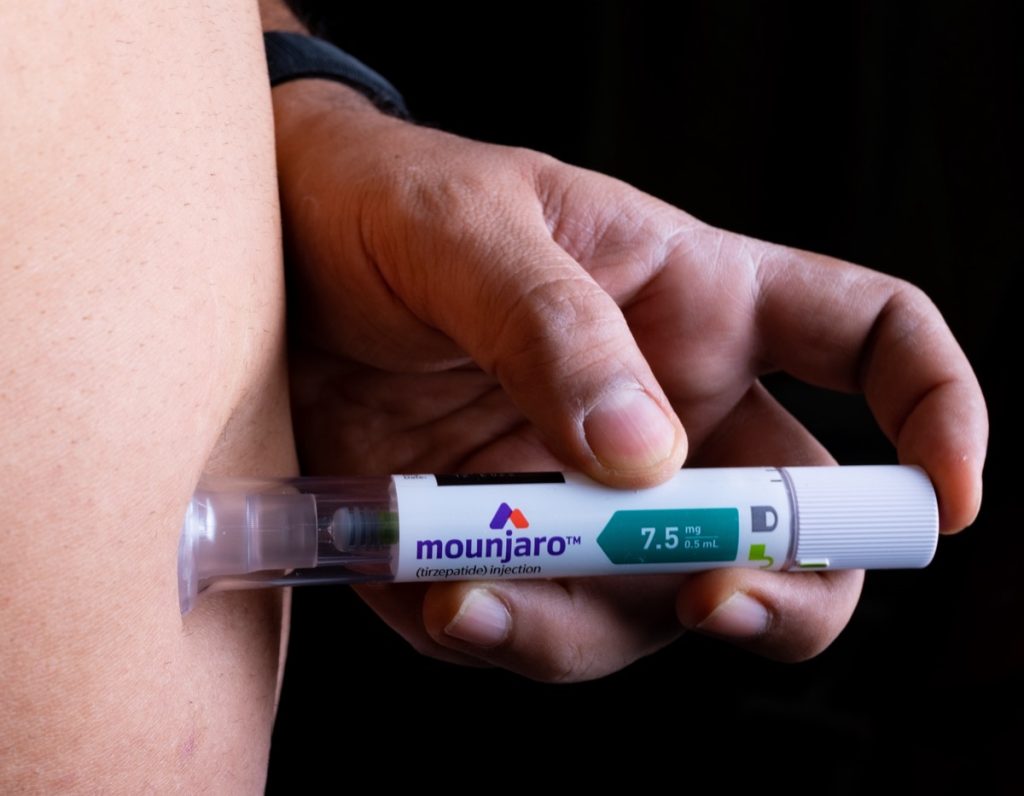
The Eli Lilly product is already on the market as Mounjaro and used to treat diabetes.

Even though the topic of weight loss has long been a part of the wellness conversation overall, recent developments have shifted the way we're approaching it. Prescription medications including Ozempic—which was approved for the treatment of diabetes and prescribed off-label for weight loss—and approved obesity drug Wegovy have changed the process of shedding pounds lately for those prescribed them. And now, the list of potential options for patients is longer after the U.S. Food & Drug Administration (FDA) approved Zepbound as a weight loss drug. Read on to learn more about the prescription medication that has been shown to slash some patients' weight by 18 percent.
RELATED: Countries Are Placing New Bans on Ozempic—Could the U.S. Follow?
Zepbound was just approved as an obesity treatment drug by the FDA.

On Nov. 8, the FDA announced it had approved Zepbound for chronic weight management and the treatment of diabetes. The drug, named tirzepatide, is produced by Eli Lilly and will compete most directly with Novo Nordisk's popular Wegovy obesity drug, The New York Times reports.
While the Zepbound name may be new, tirzepatide is already available on the market under the brand name Mounjaro, which was previously approved for treating type 2 diabetes in adults. Zepbound can be prescribed to patients who are overweight and have at least one obesity-related condition, including high blood pressure, type 2 diabetes, or high cholesterol, per the FDA's release.
Zepbound works similarly to Wegovy by mimicking the hormone GLP-1, which helps to reduce hunger and slow a person's food intake. It's also administered by injection once a week with a maximum dosage of 15 mg per week, according to the FDA.
"Obesity and overweight are serious conditions that can be associated with some of the leading causes of death such as heart disease, stroke, and diabetes," John Sharretts, MD, director of the Division of Diabetes, Lipid Disorders, and Obesity, for the FDA, said in the agency's news release. "In light of increasing rates of both obesity and overweight in the United States, today's approval addresses an unmet medical need."
RELATED: 4 Foods That Spike the Same Weight Loss Hormone as Ozempic, Experts Say.
Clinical trials have shown the drug can help patients achieve significant weight loss.
The first signs of Zepbound's effectiveness came from a small 2017 study with 300 people with type 2 diabetes. Results found that patients who took the drug for three months lost at least 13 percent of their body weight, The New York Times reports.
The following study conducted a randomized, double-blind, placebo-controlled clinical trial using 2,519 adult patients with obesity or who were overweight. Participants were given doses of either 5 mg, 10 mg, or 15 mg once a week for 72 weeks.
Results show that patients at all dosage levels who received the drug saw at least a five percent body weight reduction versus those who received a placebo, according to the FDA's release. And those in the group who received the highest dosage lost an average of 18 percent of their body weight compared to the placebo group. By comparison, patients taking a comparable dosage of Wegovy saw an average reduction in body weight of 15 percent, according to The Times.
RELATED: FDA Issues Ozempic Update After Users Cite "Severe" Gastrointestinal Issues.
The drug could help supply issues and potentially bring down the cost.

The FDA's approval also comes as the skyrocketing popularity of drugs like Ozempic and Wegovy have created shortages for patients. In fact, Novo Nordisk even recently limited lower dosages of Wegovy required for new patients so as to prioritize its supplies for those already taking the drug, CNN reports.
Zepbound's availability could also help drive down drug costs as competition increases. The medication will cost about $1,060 per month before insurance when it goes on sale later this month, according to a news release from Eli Lilly. By comparison, the company currently sets the list price for Mounjaro at $1,023 per month, while Novo Nordisk's Ozempic and Wegovy are $936 and $1,349 monthly, respectively, per CNN.
The company pointed to the 20 percent price difference between it and its competitor during its announcement, saying it had spoken to employers about payment decisions regarding employee insurance. "They said that the list price was something that was a factor in their decision to expand access to people who need these medications," Mike Mason, president of Lilly Diabetes and Obesity division, told reporters on Nov. 8, according to CNN.
RELATED: 56-Year-Old Woman Dies From Alleged Ozempic Side Effects, Family Claims.
The drug still carries some familiar side effects.

Similar to its competitors, Zepbound was still found to cause some side effects that predominantly affect the gastrointestinal system. These include "nausea, diarrhea, vomiting, constipation, abdominal (stomach) discomfort and pain," and other symptoms such as "injection site reactions, fatigue, hypersensitivity (allergic) reactions (typically fever and rash), burping, hair loss, and gastroesophageal reflux disease," according to the FDA's release.
But even as debate over the drug's usage by some patients continues, some experts see the medicine's release as a significant step in helping to combat a public health issue.
"Just a few years ago, it would be difficult to imagine two medications like semaglutide and tirzepatide that lead to weight loss that previously was only seen when people had bariatric surgery," Susan Yanovski, co-director of the office of obesity research at the National Institute of Diabetes and Digestive and Kidney Diseases, told The Times.
Best Life offers the most up-to-date information from top experts, new research, and health agencies, but our content is not meant to be a substitute for professional guidance. When it comes to the medication you're taking or any other health questions you have, always consult your healthcare provider directly.
RELATED: For more up-to-date information, sign up for our daily newsletter.